Introduction to Electrooxidation
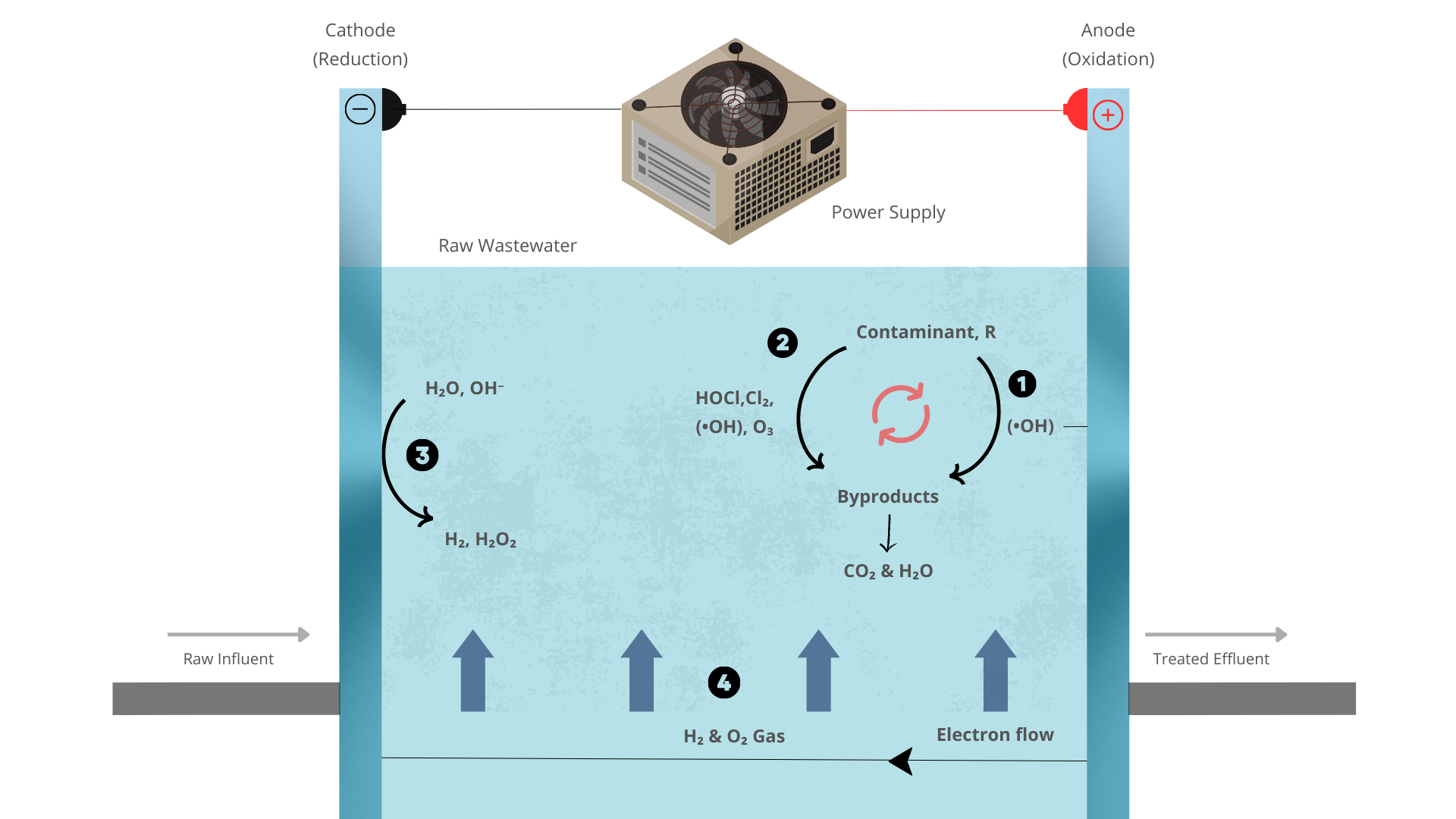
Electrooxidation is an electrochemical process that involves the oxidation of organic and inorganic compounds using an electrical current. It is a versatile technique with applications in various fields, including environmental remediation, energy conversion, and organic synthesis. This artucke aims to provide a comprehensive overview of electrooxidation, including its mechanisms, applications, and advantages and disadvantages.
The Main Mechanisms of Electrooxidation
1] Direct Oxidation
Electrooxidation occurs at an electrode surface, where the oxidation of a compound takes place through the transfer of electrons. The process involves several steps:
- Adsorption: The compound to be oxidized adsorbs onto the electrode surface and interacts with physisorbed hydroxyl radicals.
- Electron transfer: Electrons are transferred from the compound to the electrode, resulting in the formation of positive ions.
- Diffusion: The oxidized species diffuse away from the electrode surface into the electrolyte.
- Reaction with the electrolyte: The oxidized species reacts with the electrolyte, leading to the formation of final products.
The exact mechanisms of electrooxidation can vary depending on the nature of the compound and the electrode material. Common electrode materials used in electrooxidation include noble metals like platinum, as well as conductive metal oxides such as ruthenium and iridium.
2] Indirect Oxidation
In direct electrooxidation, the compound of interest is directly oxidized at the electrode surface, typically by accepting electrons from the electrode and undergoing a redox reaction. However, in indirect electrooxidation, the compound is not directly oxidized at the electrode. Instead, the electrode generates an oxidant, which then reacts with the target compound, leading to its oxidation.
The generated oxidant can be a variety of species depending on the electrochemical system and conditions. For example, in some cases, oxygen molecules from the surrounding environment can be electrochemically reduced at the electrode surface to produce reactive oxygen species (ROS) such as hydroxyl radicals (·OH) or peroxide ions (O₂²⁻). These ROS species can then diffuse away from the electrode and react with the target compound, initiating its oxidation.
Applications of Electrooxidation
- Water treatment: Electrooxidation is widely employed in water treatment processes to remove organic pollutants, such as dyes, oil and grease, pesticides, and pharmaceuticals. It offers advantages over traditional methods, as it can effectively degrade complex organic compounds, even at low concentrations, lower the hardness content of water, and eliminate pathogens such as Legionella.
- Energy conversion: Electrooxidation plays a crucial role in energy conversion devices like fuel cells and electrolyzers. In fuel cells, it is used to oxidize the fuel (e.g., hydrogen or methanol) at the anode, generating electricity. In electrolyzers, it facilitates the oxidation of water to produce hydrogen gas.
- Organic synthesis: Electrooxidation provides an environmentally friendly and efficient method for organic synthesis. It enables the selective oxidation of various functional groups, allowing the synthesis of valuable intermediates and fine chemicals. Additionally, electrooxidation can be used for the direct functionalization of organic molecules, eliminating the need for hazardous reagents.
- Waste treatment: Electrooxidation offers a promising solution for the treatment of industrial waste, including wastewater and contaminated soils. It can effectively degrade toxic and persistent compounds, reducing their environmental impact.
Advantages of Electrooxidation
- Selectivity: Electrooxidation can be highly selective, allowing specific functional groups or compounds to be targeted for oxidation. This selectivity is advantageous in organic synthesis, where precise control over reactions is essential.
- Mild reaction conditions: Electrooxidation often occurs at ambient temperatures and pressures, reducing the energy requirements and minimizing the formation of unwanted byproducts.
- Environmental compatibility: Compared to traditional oxidation methods that employ harsh and toxic chemicals, electrooxidation is environmentally friendly. It eliminates or reduces the use of hazardous reagents, making it a sustainable alternative.
How Hydroleap Optimizes Performance
- Enhanced electrode performance: In electrooxidation, careful management of electrode surfaces can lead to optimized performance. By employing effective cleaning or periodic electrode replacement techniques, the process can maintain high efficiency and ensure consistent results.
- Powerful energy utilization: Electrooxidation harnesses external power sources to drive the oxidation reaction, enabling the efficient and controlled transformation of compounds. This power-driven approach allows for precise control and customization, enhancing the overall efficacy of the process.
- Tailored electrode material selection: Electrooxidation demands a thoughtful choice of electrode materials and catalysts to maximize effectiveness. By employing specific materials suited for each compound, the process can be fine-tuned to deliver superior performance, unlocking new possibilities and ensuring optimal outcomes.
Conclusion
In total, electrooxidation is a versatile electrochemical process with numerous applications in water treatment, energy conversion, and organic synthesis. Its selective nature, mild reaction conditions, and environmental compatibility make it an attractive option for various industries. However, challenges such as electrode fouling, energy consumption, and electrode material limitations need to be addressed to optimize the process and enhance its efficiency. Continued research and development in this field hold promise for further advancements and broader implementation of electrooxidation in various sectors.


